HOW IS MILK TRANSFORMED INTO YOGURT?
2024-06-10
- Do you know how milk is transformed into yogurt?
- Why does milk curdle?
- Why does yogurt have such a different texture, flavor and smell from milk?
In this post we explain what changes occur in milk so that it becomes yogurt, and what causes them. To summarize it briefly, milk curdles and transforms into yogurt when we give it a heat treatment and then lower its pH through fermentation. We explain it in detail below.
HOW IS MILK TRANSFORMED INTO YOGURT? WHY DOES MILK CURDLE ? PHYSICAL CHANGES IN MILK
The composition of milk depends, fundamentally, on the species and breed of the animal that produces it, in addition to many other factors to a lesser extent, diet, lactation phase… In the case of cow’s milk, this milk is approximately made up of the following:
As you can see, most of milk is water, and the rest of the components are found within that water in several ways:
- In the form of an emulsion (suspension of small liquid globules that do not mix with the water in milk), as is the case with fat.
- Completely dissolved, as is the case with lactose, minerals and serum proteins
- Or in the form of a colloidal suspension, that is, a set of small solid particles that are not soluble but that are so small that they do not settle and remain dispersed in the water, this is the case of micelles, which are formed by a type of protein. milk, called caseins.
THE MICELLES OF MILK
Caseins interact with each other forming spherical particles called micelles. Micelles have the characteristic of being negatively charged on the outside, so they repel each other and remain dispersed in the water of the milk.
Effect of fermentation on micelles
When Lactobacillus and Streptococcus multiply in milk, they cause fermentation, transforming lactose into lactic acid.
This lactic acid causes the pH of the milk to go from neutral to acidic. The acidic pH causes the micelles to lose their negative charge, until there comes a time when the external charges of the micelles are neutral. At that moment they stop repelling each other and other types of attractive forces cause the micelles to join together, forming a network. Until that moment they had not manifested, due to the repulsive forces of the negative charges. The water molecules are trapped in this network, which gives rise to a coagulated fluid, more viscous than milk had been until then.
Isoelectric point
That pH at which the micelles have a neutral charge is called the isoelectric point. The isoelectric point depends on the type of casein we are talking about, there are several types, and it ranges between 4.44 and 5.77. Furthermore, it also varies with temperature. At room temperature, the average isoelectric point of caseins is reached at pH 4.6.
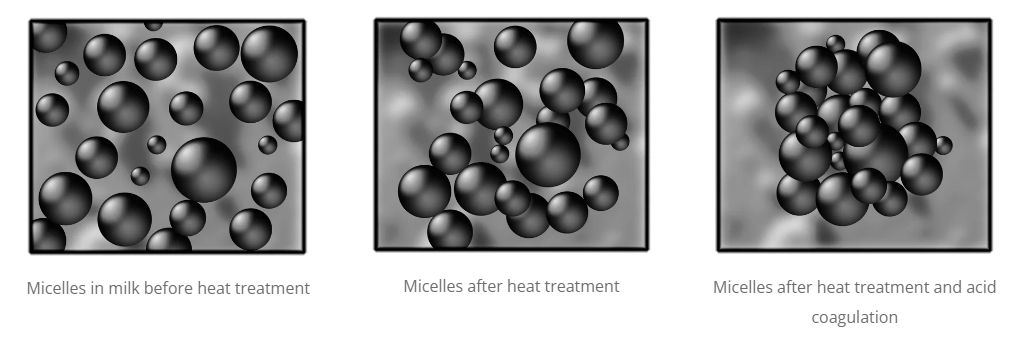
Effect of heat treatment on micelles.
How does heat treatment affect the coagulation of milk in yogurt? In addition to the process explained in the previous point, the water retention capacity of the clot and the firmness of the yogurt is increased if, before fermentation, the milk has been subjected to a heat treatment of at least 75ºC for a few minutes. This treatment causes whey proteins to be denatured, especially beta-lactoglobulin.
Denatured beta-lactoglobulins tend to bind to caseins, resulting in a firmer and smoother gel, with greater viscosity that does not present syneresis (whey exudation). You will find more information about the heat treatments that milk can receive in this article . Therefore, thanks to heat treatment and subsequent fermentation, yogurt acquires textural characteristics different from those of milk. These characteristics are usually described with terms such as: consistency, body, viscosity, softness, lumpiness… Below is a simulation of the casein micelles according to the process they have undergone.
WHAT CAUSES THE PARTICULAR TASTE AND SMELL OF YOGURT? CHEMICAL CHANGES
As yogurt bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus) multiply in milk, in addition to producing changes in the texture of the milk, they also produce changes in its smell and flavor, due to the chemical compounds they release into the milk. The main compounds that contribute to the aroma and flavor of yogurt can be grouped into four categories:

- non-volatile acids, such as lactic, pyruvic, oxalic or succinic acids;
- volatile acids, such as formic, acetic, propionic or butyric acids;
- compounds with carbonyl groups, such as acetaldehyde, acetone, acetoin or diacetyl;
- a heterogeneous group of substances, including some amino acids and/or other compounds formed by the degradation of proteins, fat or lactose due to the action of temperature.
There is general agreement in the literature that the aroma and flavor by which we recognize a food as yogurt are basically due to lactic acid and compounds with a carbonyl group, especially acetaldehyde and diacetyl.
Even though many substances are involved in the multiple nuances of aroma and flavor of yogurt, the most obvious differences in flavor and aroma between different yogurts are mainly due to the differences in acidity between them.
THE ACIDITY OF YOGURT
The acidity of a yogurt is due to the greater or lesser concentration of lactic acid in it.This acidity is expressed using the pH scale, which goes from 14, for very alkaline solutions, to 0 for very acidic solutions, with the neutral pH being 7.
In general, the higher the fermentation temperatures and the longer the fermentation times, the greater the acidity of the yogurt. The pH of yogurt, at least, should be 4.6, since that is the approximate value at which milk curdles. Once this value is reached, yogurts with a pH close to 4.6 could be defined as mild in flavor and close to 4 or lower as acidic yogurts. As a general recommendation, pH values between 4.5 and 4 are usually sought, in which complete curdling has occurred and the best aromas have been achieved without becoming excessively acidic. (But it’s all a matter of taste)
Below pH 4.5, much more fermentation time is needed to continue decreasing the pH.
This makes yogurt making an easy and flexible process, in which you don’t have to be too worried about the yogurt becoming excessively acidic if we overdo the fermentation time. This can be seen in the following indicative graph, which shows the evolution of the pH of the yogurt over time, depending on the fermentation temperature.
Knowing the theory of acidity allows us to know that simplicity in practice is possible.
As a guide, it could be said that yogurt fermenting at temperatures close to 40ºC will be done in about 6 to 8 hours. Once you become familiar with this process, which doesn’t take long, you intuitively know when to remove the yogurt from the yogurt maker and put it in the refrigerator to obtain a level of acidity to your liking.
And even more importantly, he knows that if the time comes he forgets to pick up the yogurt for a while… that won’t be a big deal either. Furthermore, there is no excess acidity that a little honey cannot correct.




